
Are you having problem with rust / corrosion? Yes, it is a typical “metal problem” that you wish to avoid. In this article, the author, who has 13 years of experience in procuring metal parts and materials for Toyota Motor Corporation and startup companies, will introduce the following with my experiences.
- Rust and corrosion are phenomena in which metals are degraded or damaged by interaction with the external environment, the main factor being oxidation reactions. Rust is a type of corrosion.
- There are 7 types of corrosion, each with different causes.
- Corrosion can be counteracted by: identifying (and in some cases accepting) the effects of corrosion, avoiding it through mechanical design, changing the material, and treating it with corrosion inhibitors.
Corrosion is a phenomenon in which metals are degraded or damaged by the interaction of metals with the external environment, mainly caused by oxidation reactions. Metal surfaces, react with oxygen, moisture and chemicals. The metal surfaces are covered by oxides and chlorides, which deteriorate and damage the metal. Corrosion can be a serious problem for metal products and structures.
Some metals such as Iron is chemically unstable on its own. Therefore, Iron combines with oxygen etc to form compounds such as oxides etc and becomes stable (reference to the below image).
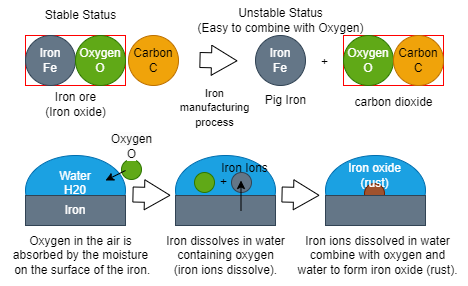
Like the image above, when metals come into contact with water, they ionize and become more reactive with oxygen. It then combines with oxygen in the air and begins to oxidize or form rust = corrosion. In other words, metals that ionize easily are more likely to rust. The oxidation reaction (corrosion) that occurs when metals are exposed to moisture or liquids is known as wet corrosion.
On the other hand, if corrosion occurs without the involvement of liquids, it is called dry corrosion.
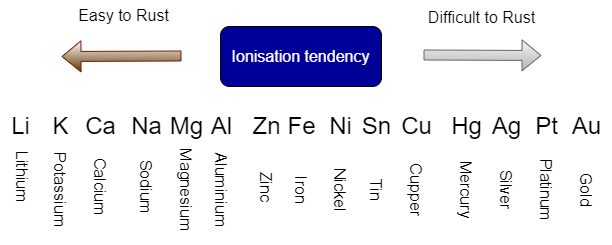
The ionization tendency of metals is illustrated in the diagram below. The ionization tendency is like metal corrosion ranking. Basically, metals with a higher ionization tendency, such as Lithium (Li) and Potassium (K), rust more easily, while metals with a lower ionization tendency, such as Gold (Au) and Platinum (Pt), rust less easily.
Corrosion is the reaction of metals when a metal reacts with another substance such as oxygen, hydrogen, an electrical current or even dirt and bacteria. Corrosion can also happen when metals like iron are placed under too much stress causing the material to crack.
In conclusion, rust is one kind of corrosion. From now on, we refer to the word “corrosion” as an inclusive word.
Above ionization tendency indicates that Fe (Iron) is easier to rust / corrode than cupper. Although aluminum rusts more easily than iron, when exposed to air, aluminum rusts immediately and its surface becomes aluminum oxide. The surface is then covered with a film of aluminum oxide, which prevents further rusting. The aluminum oxide film is resistant to use and looks beautiful, but it is already rusted.
There are different kinds of corrosion. We introduce 7 corrosion types here.

General corrosion is a form of corrosion that proceeds uniformly across the entire surface of the metal. General corrosion occurs in environments with weak oxidizing acids such as hydrochloric acid, sulphonic acid, phosphoric acid and organic acids. In such use cases, considering other metal is recommended.

Local corrosion is another form of corrosion that proceeds in parts of the surface of the metal. It is caused by localized breakdown of the passive film by the action of chlorine ions, etc., and progresses by preferential destruction of that part of the film. Pitting corrosion occurs on the surface in the form of spot or worm-eaten corrosion. Crevice corrosion occurs in the gaps between metals. To avoid local corrosion, you can consider the following,
-eliminating crevice structures
-select materials for high corrosion resistance

Intergranular Corrosion is a kind of local corrosion in which corrosion occurs preferentially at the grain boundaries of a metallographic structure. Intergranular Corrosion is caused sensitization (the combination of chromium and carbon caused by welding, high heat environment. the combined chromium carbides are formed, the amount of chromium decrease, resulting in susceptible to corrosion). To avoid sensitization, you can take following counter measures,
-avoiding heat environment or heat concentration
-conducting a special process called “solution heat treatment” for stainless steels
-selecting materials of less sensitization risk

SCC occurs under the action of corrosion factors such as chloride ions and tensile stress. SCC is typical for austenitic stainless steels such as 304. A typical SCC takes place on the swimming pool structural materials. To avoid SCC, you can consider the followings,
-reducing concentration of corrosion factors
-reducing tensile stress
-selecting other materials (e.g., ferritic stainless steel)

Galvanic corrosion is a kind of corrosion due to chemical reactions of combining different metals.
When combining two different metals, the metal with the greater ionization tendency becomes the anode (+ electrode) and the one with the lesser ionization tendency becomes the negative electrode (- electrode). The anode side is corroded by potential transfer and loss of charged atoms or molecules (=ions).
I had an experience of auto parts having galvanic corrosion for steel + aluminum with catalysis of anti-icing agent and snow.

High Temperature Oxidation occurs when metal surfaces react with oxygen in high-temperature environments to form oxides.
A typical example is Hot Rolled Coil. Hot Rolled coils have black scale (oxide film) caused by High Temperature Oxidation. Steel mills usually have pickling or griding processes to remove the black scale. Since high-temperature oxidation is inevitable in the manufacturing process, steelmakers usually have pickling and grinding processes to remove the black film.

High Temperature Corrosion occurs when metal react with chemical gases or combustion products, causing metal surfaces to corrode. High-temperature gases contain corrosive components such as oxygen, nitrogen, sulfur, and chlorine. When the metal reacts with these components, a corrosion layer is formed on the metal surface and erodes into the metal interior.
A typical example is Corrosion in offshore rigs and vessels due to handling seawater in high temperature environments. Countermeasures include selecting metals that are resistant to high-temperature corrosion (heat-resistant alloys and heat-resistant steels) and not neglecting periodic maintenance.
There are several methods to prevent corrosion such as mechanical design change, material change, and anti-corrosion treatment.
Before taking measurement of corrosion prevention. It is good to step back and reconsider the impact / consequences of corrosion. Corrosion is generally considered to be harmful in terms of metal’s performance or appearance. Often, the consequences of corrosion is negative. However, some corrosion could be accepted. For example, brake discs of cars and motorbikes are easily corroded. Brake discs in cars are made of a material called “cast iron”, which is less prone to distortion during the cooling process after reaching a high temperature and has a relatively stable coefficient of friction with the disc pads. Corrosion is performance-wise tolerated because rust is worn away by friction with the brake pads. Corrosion is also appearance-wise tolerated because corrosion on disc brakes are less visible as disc brakes are covered by wheels.

In case you judge corrosion prevention is necessary, planning ahead to prevent corrosion is a good idea. Designing metal components to avoid cracks and pits where water enters or stays in a certain spot is very effective to prevent corrosion. Also, keeping good ventilation is effective to prevent corrosion. Facilitating inspection and replacement through regular maintenance is even more effective. For example, at the bottom of car doors, there are holes to drain water between door panels.

Choosing material of less ionization such as cupper or cupper alloy or stainless steel is one solution. For example, airplanes use aluminum bronze (copper alloy) for landing gears because aluminum bronze is strong and durable, yet also flexible enough to withstand the stresses of landing and takeoff. Aluminum bronze is also resistant to corrosion and wear, which helps to extend the lifespan of the landing gear. Of course, copper alloy material cost is higher so that you may need to consider not only the mechanical and chemical properties, but also the balance between the material cost and material life plus maintenance costs.
Supplementally, aluminum is generally resistant for corrosion by itself. Aluminum can be easily rusted on surface, forming a film of aluminum oxide. This film prevents further rusting. However, if aluminum is connected with iron and constantly exposed to water, a special type of corrosion called “galvanic corrosion” may take place. In this case, aluminum can be corroded inside easily. Galvanic corrosion occurs between metals with widely different ionization tendencies through water, so it can also occur between copper and iron, copper and aluminum, and so on.

For corrosion-prone metals, metals can be treated to make them less susceptible to corrode. This treatment is called anti-corrosion treatment. The most commonly used anti-corrosion treatment is to form a film on the surface of the metal with another substance, which prevents the metal from being exposed to oxygen and water in the air. Anti-corrosion treatment is classified into organic and inorganic coatings, depending on the material of the coating.

Organic coating is typically painting for irons. Oil coating or covering with a rubber or plastic film, such as a conductor, is also a form of corrosion protection using an organic film. This type of coating is easy and often more visible while removal of such coating on the surface immediately looses the coating effect.

Inorganic coating is typically plating for irons. Plating is a method of forming a film of another metal on the surface of a metal material. For example, a metal that forms a passive film, such as chromium, or a metal that has the property of protecting the interior by rusting before the metal inside, such as zinc. Chemical conversion coatings are also often used. Chromate treatment and anodized aluminum are examples of methods that use chemicals to form a film that is resistant to oxidation and corrosion.
This type of coating also corrodes and the underlying iron is exposed until the electrochemical corrosion protection of the coating is no longer available, the iron will start to corrode.
For any method you apply, we encourage you to make sure that the metal is clean before surface treatment. There are many simple mistakes that steel is painted without removing black scale and rust.
Overall, corrosion is frequent concerns for any products made of metals because metals such as iron can be oxidize easily. Measurement of corrosion can be basically 4 ways as reconsidering the impact of corrosion, mechanical design change, material change, and anti-corrosion treatment. From Special Steel & Stainless, Copper Alloys line ups, Enserve is happy to help you solving your problems by establishing tailor-made supply chain solutions.


